
Jeremy Mathews
Research Project Manager
Office: 312.503.3753 | Pager: 312.695.8675
jmathews@northwestern.edu
The PCF Bio-repository laboratory specializes in the procurement, processing, preservation and distribution of biospecimens collected for research purposes. Patient and biospecimen information is collected and stored using the BSI-II (Biological Specimen Inventory-II) system which provides a secure software environment for the date entry, storage, requisitions, shipment tracking and reporting of specimens. Biospecimens are physically stored securely within our -80°C freezers, various cold rooms and lab space. Our freezers and refrigerators are monitored using the Mesa Amega View continuous monitoring system, which is designed to safe guard the specimens and notify staff of any potential issues. The biorepository currently has 3 full time employees (FTE) which consist of two research study coordinators which handle patient screening, consenting, fluid collection, processing, data entry specimen storage and distribution. Also one Pathology Assistant (PA) which handles tissue grossing and collection.
An organized specimen collection, for example for the Prostate Spore requires high degree of coordination with other departments including the Northwestern Memorial Hospital, clinics and bioinformatics. The PCF technicians and coordinators strategically collect biospecimens without compromising patient care. PCF ensures that NMH Surgical Pathology obtains sufficient material for clinical diagnosis prior to transfer to research for immediate processing, assessment of the specimen, preservation and storage.
PathCore uses a web-based tool, Disburser, for submitting and managing disbursement requests for specimen and/or data from biorepositories across Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Disburser User Guide »
For more information, see our poster.
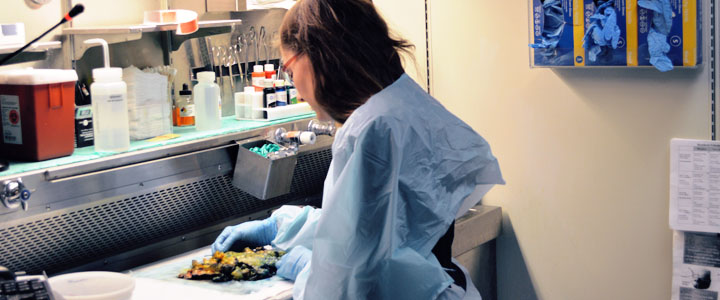
We receive consented patient's specimens in the Pathology gross room and handle the specimen for routine processing which includes but is not limited to measuring, weighing, photographing, inking margins, sectioning, examining the tissue, and dictation. Once this task is complete, we then proceed to take tissue sections for patient diagnosis and in the same process we also collect the approved material stated in the CSRC (Clinical Specimen Release Committee) protocol for research. Depending on the quantity of tissue collected, the tissue is divided into two for future RNA usage and prepared as frozen tissue blocks (snap frozen in liquid nitrogen). Both of these pieces are labeled with a unique Path Core number and retained in a -80°C freezer till tissue is requested. A part of the tissue sample is also placed in 10% formalin and submitted to Path Core Histology for an H&E stain.
Pathology Core Facility
of the Robert H. Lurie Comprehensive Cancer Center
Northwestern University
Olson Pavillion, 710 North Fairbanks Court, Chicago
8th Floor, Room 8-419
312.908.5546
FAX: 312.503.1148